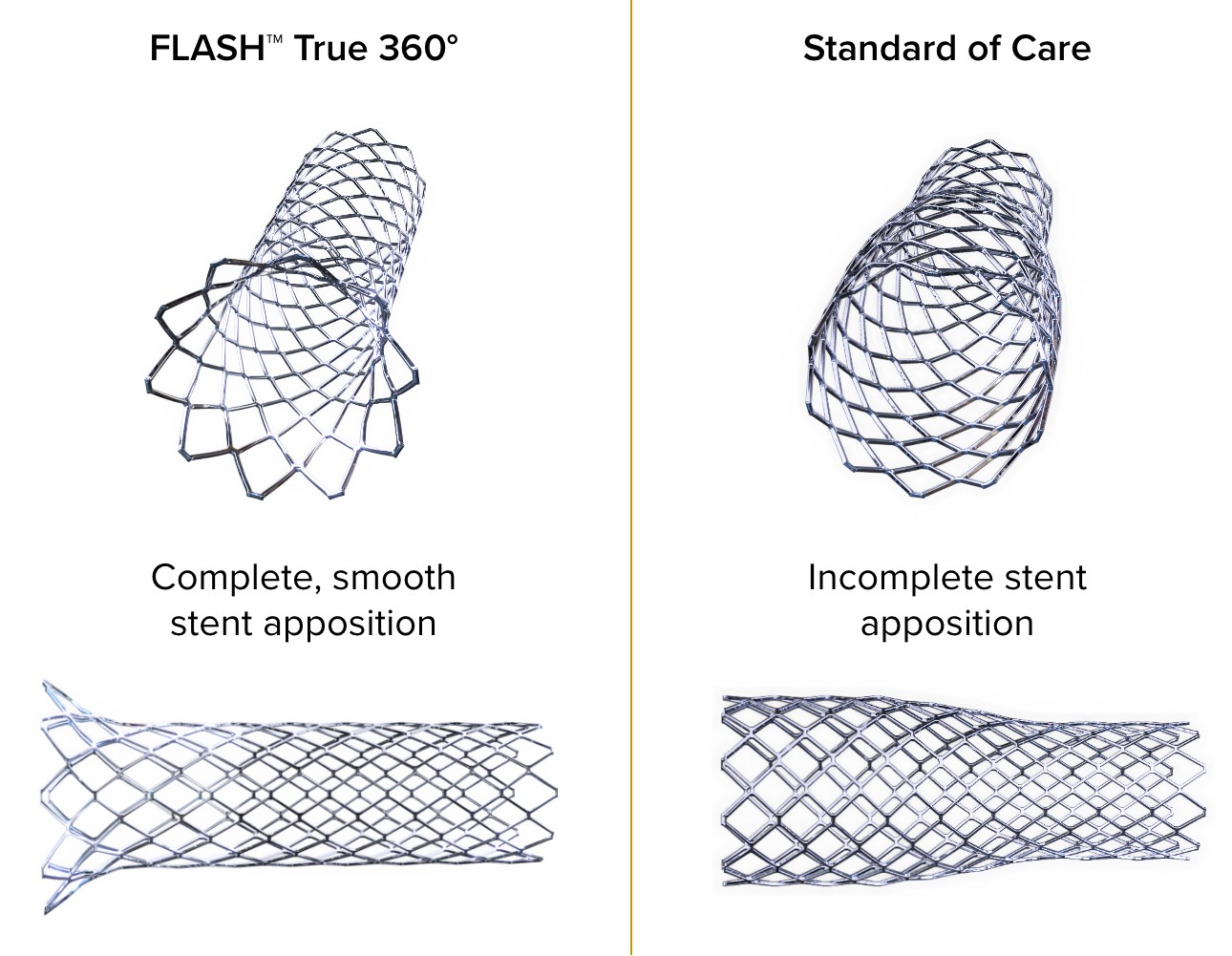
Only FLASH™ achieves total aorto-ostial stent apposition
- Optimize Patient Outcomes
- Total & Efficient Lesion Coverage
- Streamlined Vessel Reaccess
Only FLASH™ achieves total aorto-ostial stent apposition
- Optimize Patient Outcomes
- Total & Efficient Lesion Coverage
- Streamlined Vessel Reaccess
The Challenge
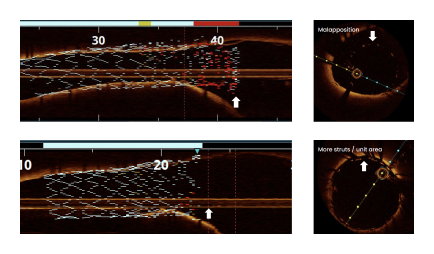
Suboptimal Lesion Coverage
Standard PTCA catheters are unable to create a smooth transition at the ostium creating suboptimal lesion coverage, which is associated with poor outcomes.1
The solution
97%
of lesions demonstrated complete stent apposition2,3
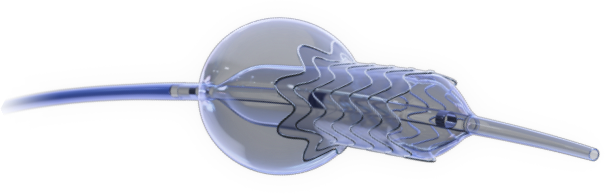
Novel Dual-Balloon Technology
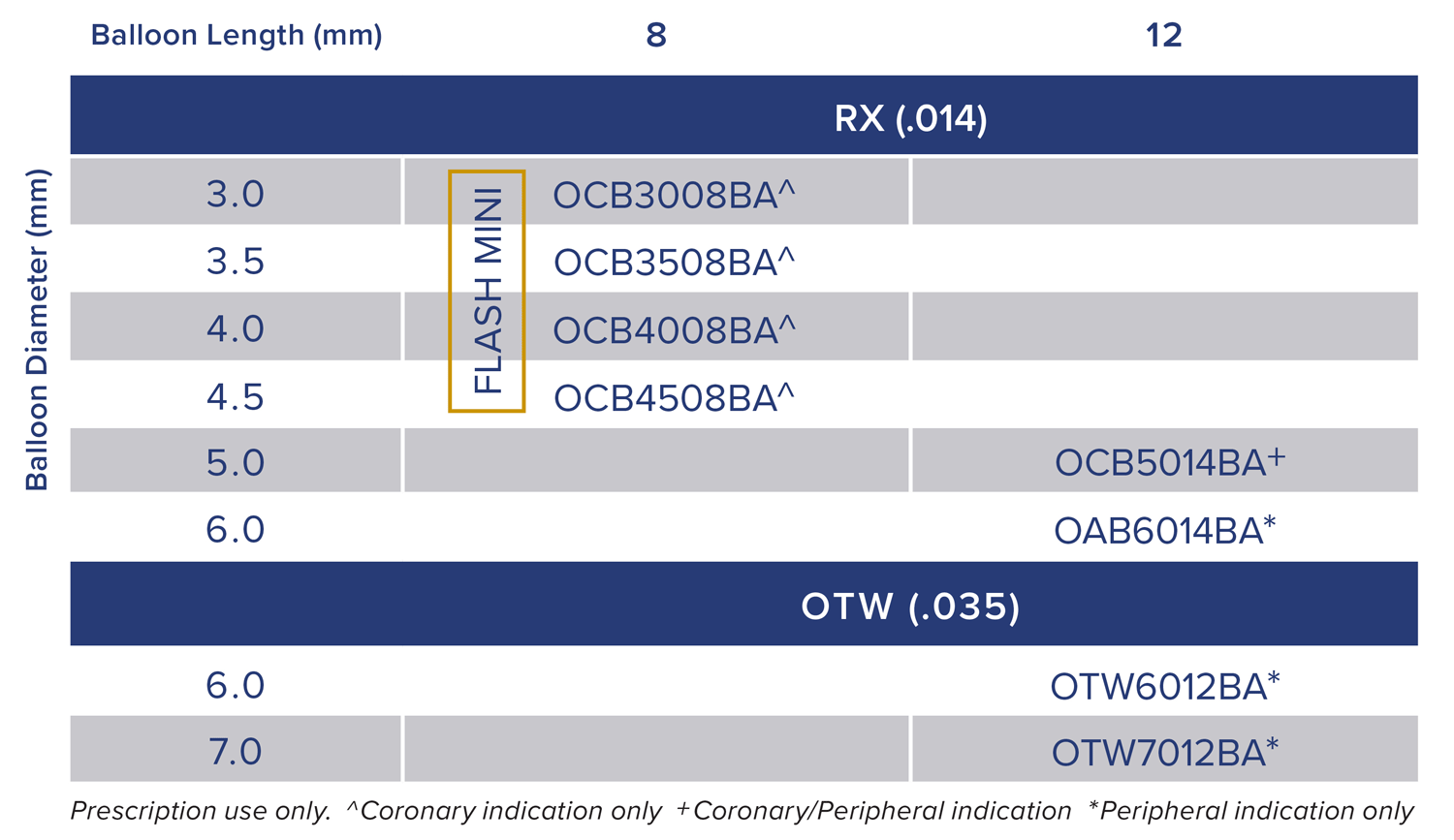
Available for both coronary and peripheral indications.
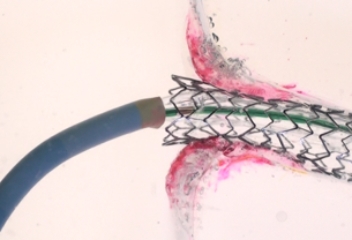
EASY DELIVERY AND CATHETER POSITIONING
The FLASH system is designed to confidently access and address challenging aorto-ostial stenting, providing total lesion coverage.
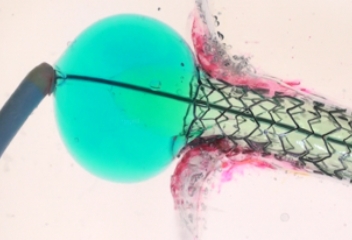
TRUE 360° FLARED APPOSITION
Novel 2-in-1 dual balloon design allows for inflation of a non-compliant distal balloon to anchor the system, while inflation of the compliant, low-pressure proximal balloon conforms the stent to the wall of the ostium.
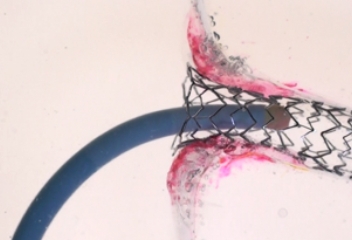
STREAMLINED
REACCESS
With TRUE 360° wall apposition achieved, a smooth transition is created for improved reaccess in future interventions.1,2
confident outcomes
The FLASH System demonstrates consistently low restenosis at 6 months, below the 9% rate reported for accurate stent placement in ostial lesions.6§
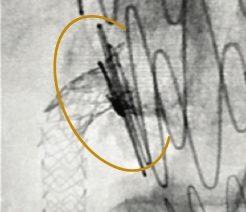
Excellent Final Result
RCA Ostial Stenosis
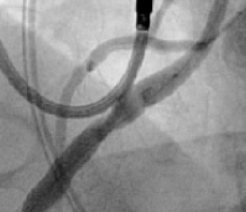

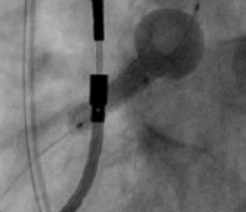
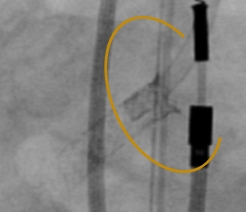
FEVAR
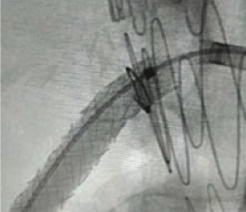
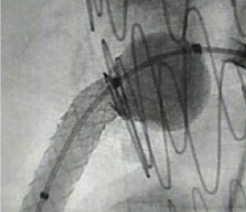
Resources
Case studies, training videos, and more.
About

Mike Buck
Chief Executive Officer

Michael Lankford
Vice President of Sales

Jon Bohane
Chief Operating Officer

Janet Shahbazi
Head of Quality

Sam Hesami
Corporate Controller
newsroom
Verge Medical, a privately held medical technology company focused on advancing physician-driven solutions to address persistent gaps in vascular care, today announced the acquisition of the RoVo™ System and its patented Temporary Occlusion Embolectomy (TOE) technology from 2MG Medical. The acquisition enhances Verge’s expanding portfolio of targeted, setting-specific solutions for coronary and peripheral vascular interventions.
Read MoreOstial Corporation, a privately held medical technology company focused on addressing the clinical challenges of aorto-ostial interventions, today announced the acquisition of the Wavella™ technology from Crossfire Medical. The company also unveiled its new identity as Verge Medical, marking a strategic expansion in the coronary and peripheral markets, with product offerings now in the inpatient and office-based settings.
Read MoreOstial Corporation (Ostial), a private medical technology company focused on addressing the clinical challenges of aorto-ostial interventions, is pleased to announce the appointment of Mike Buck as its new Chief Executive Officer. Mike brings over 30 years of cardiovascular experience, having held several executive leadership positions at prominent companies, including Guidant, Abbott, Cardinal Health, and most recently, TRUVIC Medical.
Read MoreProceeds to accelerate commercialization and pipeline expansion of novel FLASH™ Aorto-Ostial Angioplasty System
Ostial Corporation (Ostial), a private medical technology company focused on addressing the clinical challenges of aorto-ostial interventions, today announced the raise of $7.5M in growth funding led by Delos Capital with participation from AMED Ventures and other existing investors.
Ostial Corporation (Ostial), a private medical technology company focused on addressing the clinical challenges of aorto-ostial interventions, today reached a new milestone, passing the mark of 15,000 commercial units sold of its FLASH™ Aorto-Ostial Angioplasty System (FLASH System) in over 300 hospitals across the United States. FLASH is the first and only dual balloon-based system designed for use in aorto-ostial angioplasty in coronary and peripheral cases.
Read MoreFind out more about FLASH™
§compared to non-ostial lesions
- Cook, et al. Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation European Heart Journal 2012. 33:1334-1343.
- Data on file at Ostial Corporation.
- Sanghvi K, Morris T, Kovach R. Optimal Aorto-ostial Lesion Treatment With Flash Ostial Balloon Is Feasible and Safe. JACC. 2016;9(4). Supplement S.
- Nguyen-Trong P, Martinez Parachini J, Resendes E, et al. Procedural Outcomes With Use of the Flash Ostial System in Aorto-Coronary Ostial Lesions. Catheter Cardiovasc Interv.
2016;88(7):1067-1074. - Data on file at Ostial Corporation.
- Dishmon D, Elhaddi A, Packard K, et al. High Incidence of Inaccurate Stent Placement in the Treatment of Coronary Aorto-Ostial Disease. JIC. 2011;23(8).
Indications, Contraindications, Warnings & Precautions
The FLASH™ Ostial system in a specialty balloon device and should only be used by physicians who are trained to use it.
To request a complete copy of the IFU or user training, please contact customer service at customerservice@ostialflash.com.